Site-Specific Modification of Recombinant Proteins
The site-specific modification of recombinant, expressed proteins remains a challenge of great contemporary interest. While powerful approaches including cysteine engineering and the introduction of non-canonical amino acids are suitable for many applications, the preparation of homogenous conjugates of therapeutic proteins or the site-specific conjugation of ubiquitin chains require new solutions that can operate on native, folded proteins.
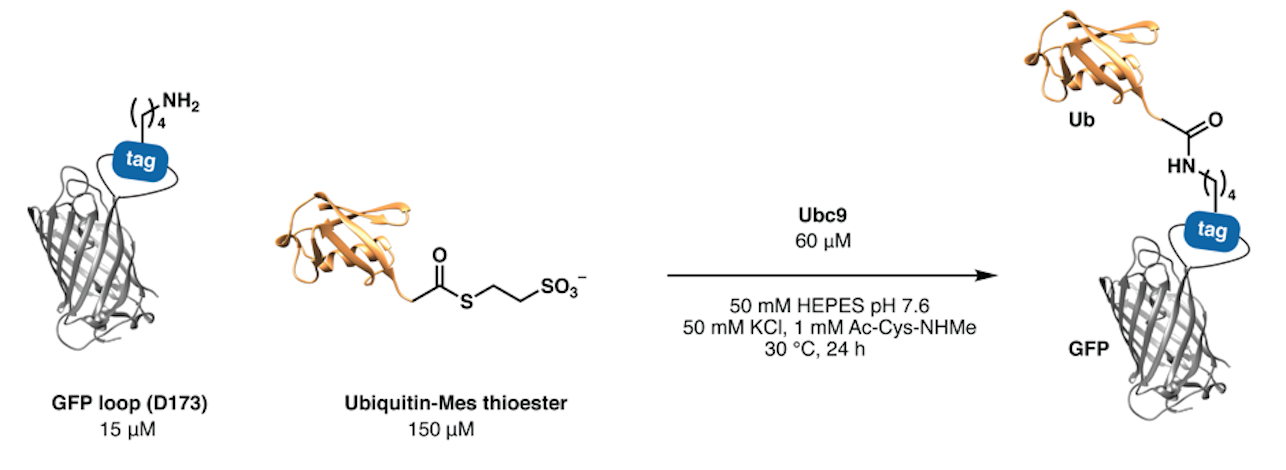
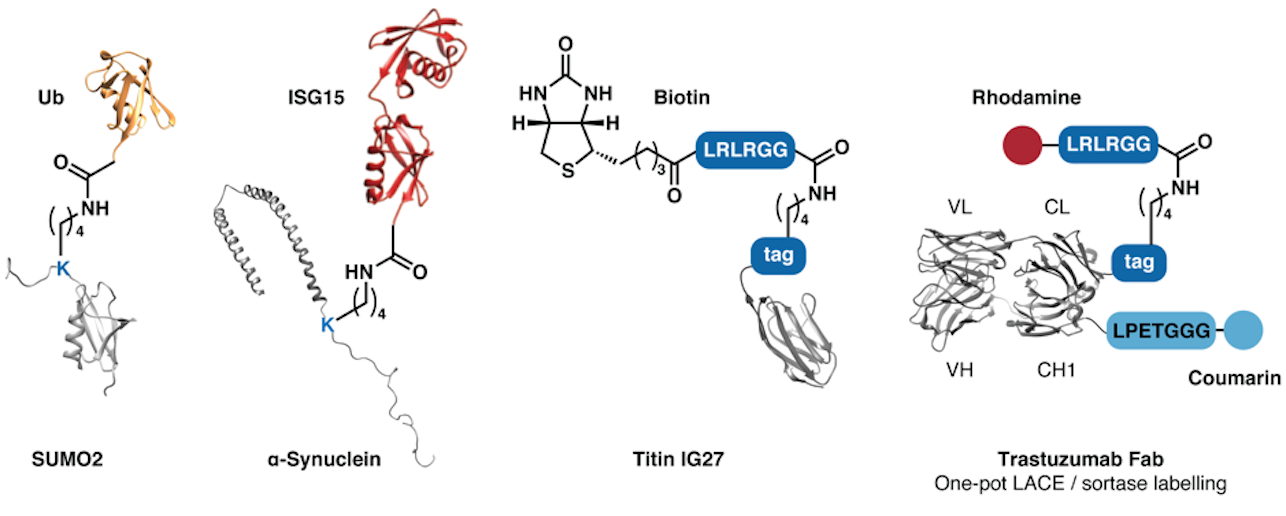
By building on our experience gained from the synthesis of E2 ubiquitin-conjugating enzymes, we identified a chemoenzymatic approach to attach peptides and proteins to a distinct lysine residue of recombinant proteins bearing a concise four-residue tag (IKXE). This method – called Lysine Acylation by Conjugating Enzymes (LACE) – enables the preparation of protein–protein conjugates with unique topologies from expressed protein targets and synthetic or expressed thioesters. It uses the SUMO conjugating enzyme Ubc9, which is commercially available or readily expressed and isolated. Applications include the site-specific labelling of proteins with dyes, biotin, or ligation handles, and site-specific ubiquitination and ISG15ylation. The methods can be easily combined with other chemoenzymatic labelling methods including sortase for dual-labelling of expressed proteins.
Examples:
We are currently exploiting the power of LACE to generate new modalities of therapeutic proteins, provide access to ubiquitinated proteins, and generate faster variants using directed evolution. We are also exploring other approaches to site-specific protein modification, with an eye to practical strategies that can be easily implemented and utilized. MSc thesis students, PhD students and postdocs with experience in biochemistry and molecular biology or an interest in learning these techniques are welcome!
Reference:
Hofmann, R.; Akimoto, G.; Wucherpfennig, T. G., Zeymer, C., Bode, J. W.;external page "Lysine Acylation Using Conjugating Enzymes (LACE) for Site-Specific Modification and Ubiquitination of Native Proteins", Nat. Chem. 2020, 12, 1008–1015.