Synthetic Fermentation
The beauty of microbially produced natural products is that complex, stereochemically rich, biologically active molecules can be grown in aqueous media without organic solvents, reagents, or toxic byproducts.
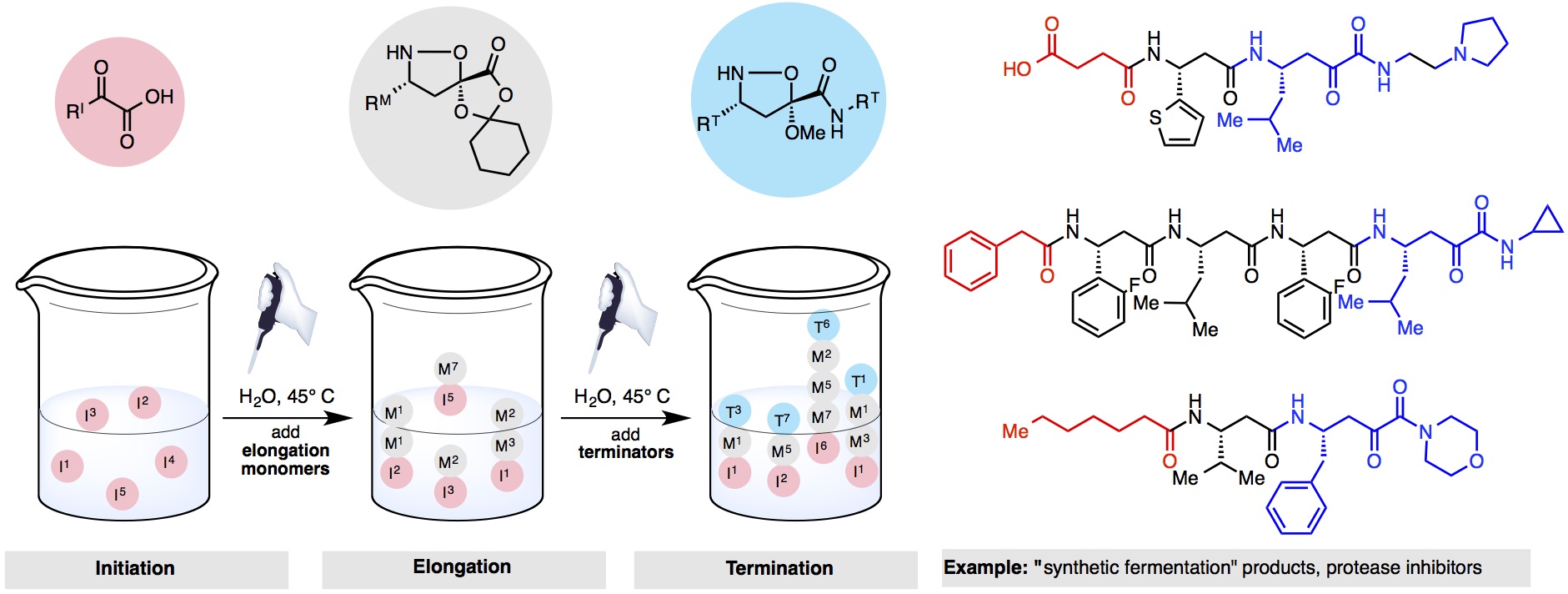
Inspired by microbial fermentation, we have developed “synthetic fermentation,” a technique where small molecule building blocks can be coupled together in aqueous media, with no organisms, enzymes or reagents, to furnish unprotected organic molecules. These “cultures” can be used directly in biological assays. Thousands of molecules can be “grown” in aqueous media in a few hours using nothing more than a pipette and a multi-well plate.
Our best implementation produces β-peptide oligomers using variants of our KAHA and KAT ligation, As a proof-of-concept, this technique has already been used to identify an inhibitor of hepatitis C virus NS3/4A protease with a half-maximum inhibitory concentration of 1.0 μM. Work is currently ongoing on further therapeutic applications, such as the discovery of new antibiotics, as well as broadening the scope of the technique through product diversification and expansion of the building block library.
Notable publications:
(97) Huang, Y.-L.; Bode, J. W. "external page Synthetic fermentation of bioactive non-ribosomal peptides without organisms, enzymes or reagents", Nature Chem. 2014, 6, 877–884.
(146) Hubert, J. G.; Stepek, I. A.; Noda, H.; Bode, J. W.; external page "Synthetic Fermentation of β-peptide Macrocycles by Thiadiazole-Forming Ring-Closing Reactions" Chem. Sci. 2018, 9, 2159–2167
(149) Review article: Stepek, I. A.; Bode, J. W.; external page "Synthetic fermentation of bioactive molecules" Curr. Opin. Chem. Biol. 2018, 46, 18–24
(166) Stepek, I. A.; Cao, T.; Koetemann, A.; Shimura, S.; Wollscheid, B.; Bode, J. W.; external page "Antibiotic discovery with synthetic fermentation: library assembly, phenotypic screening, and mechanism of action of beta-peptides targeting penicillin-binding proteins" ACS Chem. Biol. 2019, 14, 1030-1040