Acylboronates for amide-forming bioconjugation
A major limitation of many chemoselective reactions – including our own KAHA ligation – is relatively slow reaction rate. This prevents the conjugation of large molecules under the dilute (1 mM or less) concentrations suitable for their solubilization and handling. An ideal ligation reaction for synthetic applications such as protein–protein conjugation would employ stable, easily introduced functional groups and proceed under aqueous conditions at room temperature with a second order rate of 10 M-1 s-1 or faster. [104]
To meet this challenge, we have identified potassium acyltrifluoroborates (KATs) as unique, stable functional groups that undergo rapid amide-forming ligations with hydroxylamines. [67,90] These ligations proceed in aqueous media, in the presence of fully unprotected functional groups, with rate constants up to 100 M-1 s-1, depending on the structure of the KAT and reaction pH. We have established several new methods for the synthesis of KATs and other acylboronates and their incorporation into peptides, polymers, proteins, and small molecules, including N-heterocycles.
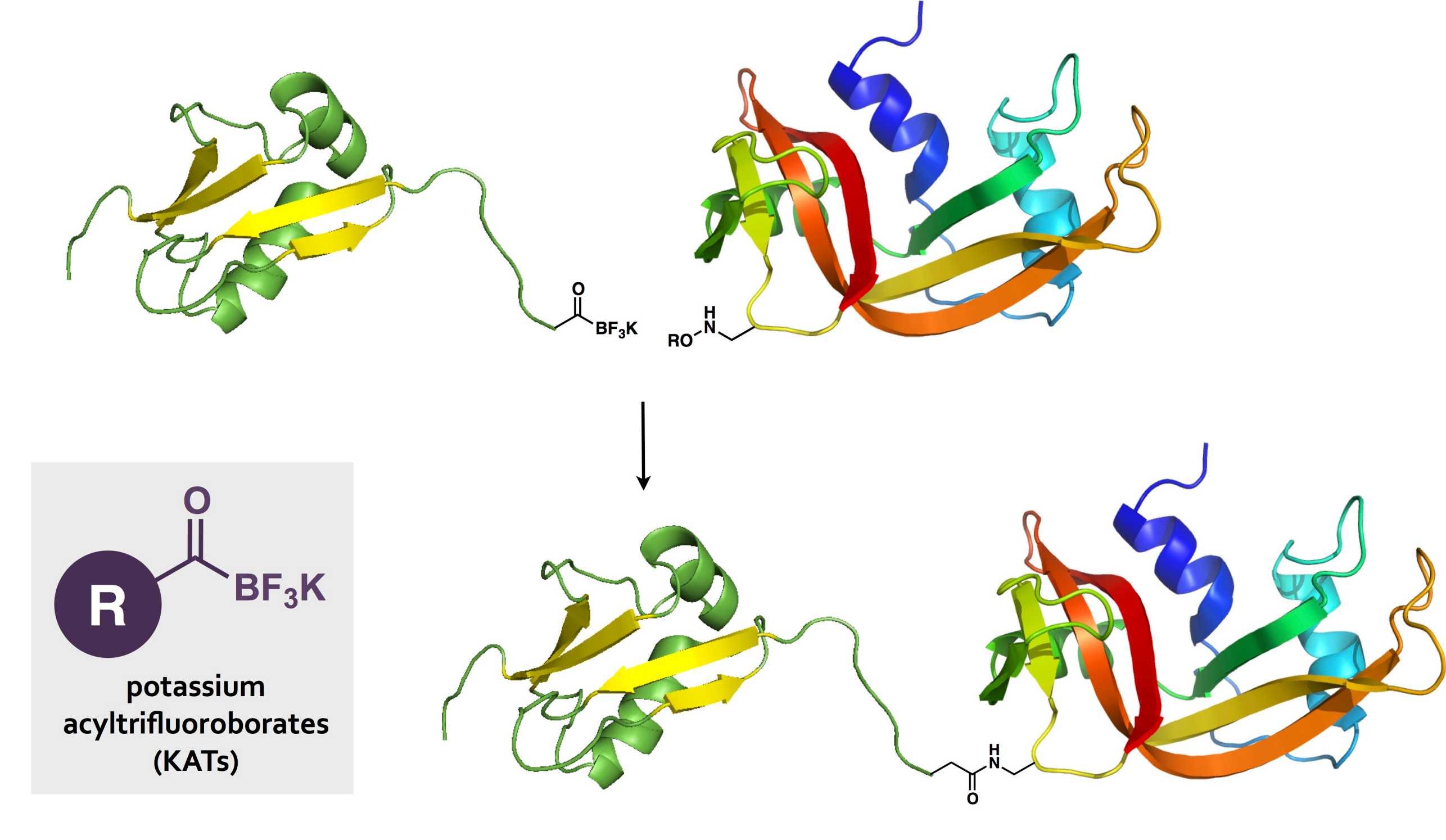
This conjugation reaction provides a new approach to the synthesis of complex molecules such as protein-protein, protein-polymer, and protein–small molecule conjugates. The KAT ligation also serves as an ideal reaction for the formation of hydrogels. We are currently studying the application of KATs within fields such as protein chemistry and material science, emphasizing the versatility of these molecules and the opportunity for even greater breakthroughs yet to come.
Notable Publications:
(66) Dumas, A. M.; Bode, J. W. external page "Synthesis of Acyltrifluoroborates", Org. Lett. 2012, 14, 2138–2141.
(67) Dumas, A. M., Molander, G. A., Bode, J. W. external page "Amide-Forming Ligation of Acyltrifluoroborates and Hydroxylamines in Water", Angew. Chem. Int. Ed. 2012, 51, 5683–5686.
(90) Noda, H; Eros, G; Bode, J. W. external page "Rapid Ligations with Equimolar Reactants in Water with the Potassium Acyltrifluoroborate (KAT) Amide-Formation", J. Am. Chem. Soc. 2014, 136, 5611–5614.
(95) Eros, G.; Kushida, Y.; Bode, J. W. external page "A Reagent for the One-Step Preparation of Potassium Acyltrifluoroborates (KATs) from Aryl- and Heteroarylhalides", Angew. Chem. Int. Ed. 2014, 53, 7604–7607.
(104) Saito, F.; Noda, H.; Bode, J. W. external page "Critical evaluation and rate constants of chemoselective ligation reactions for stoichiometric conjugations in water", ACS Chem. Biol. 2015, 10, 1026–1033.
(126) Liu, S. M.; Mazunin, D.; Pattabiraman, V. R.; Bode, J. W.; external page "Synthesis of Bifunctional Potassium Acyltrifluoroborates" Org. Lett. 2016, 5336–5339
(132) Mazunin, D.; Bode, J. W. external page "Potassium Acyltrifluoroborate (KAT) Ligations are Orthogonal to Thiol-Michael and SPAAC Reactions: Covalent Dual Immobilization of Proteins onto Synthetic PEG Hydrogels" Helv. Chim. Acta 2017, 100, e1600311
(135) Osuna Gálvez, A.; Schaack, C. P.; Noda, H.; Bode, J. W. external page "Chemoselective acylation of primary amines and amides with potassium acyltrifluoroborates under acidic conditions" J. Am. Chem. Soc. 2017, 139, 1826–1829
(145) White, C. J.; Bode, J. W.; external page "PEGylation and Dimerization of Expressed Proteins under Near Equimolar Conditions with Potassium 2-Pyridyl Acyltrifluoroborates" ACS Cent. Sci. 2018, 4, 197–206
(148) Liu, S. M.; Wu, D.; Bode, J. W.; external page "One-Step Synthesis of Aliphatic Potassium Acyltrifluoroborates (KATs) from Organocuprates" Org. Lett. 2018, 20, 2378–2381