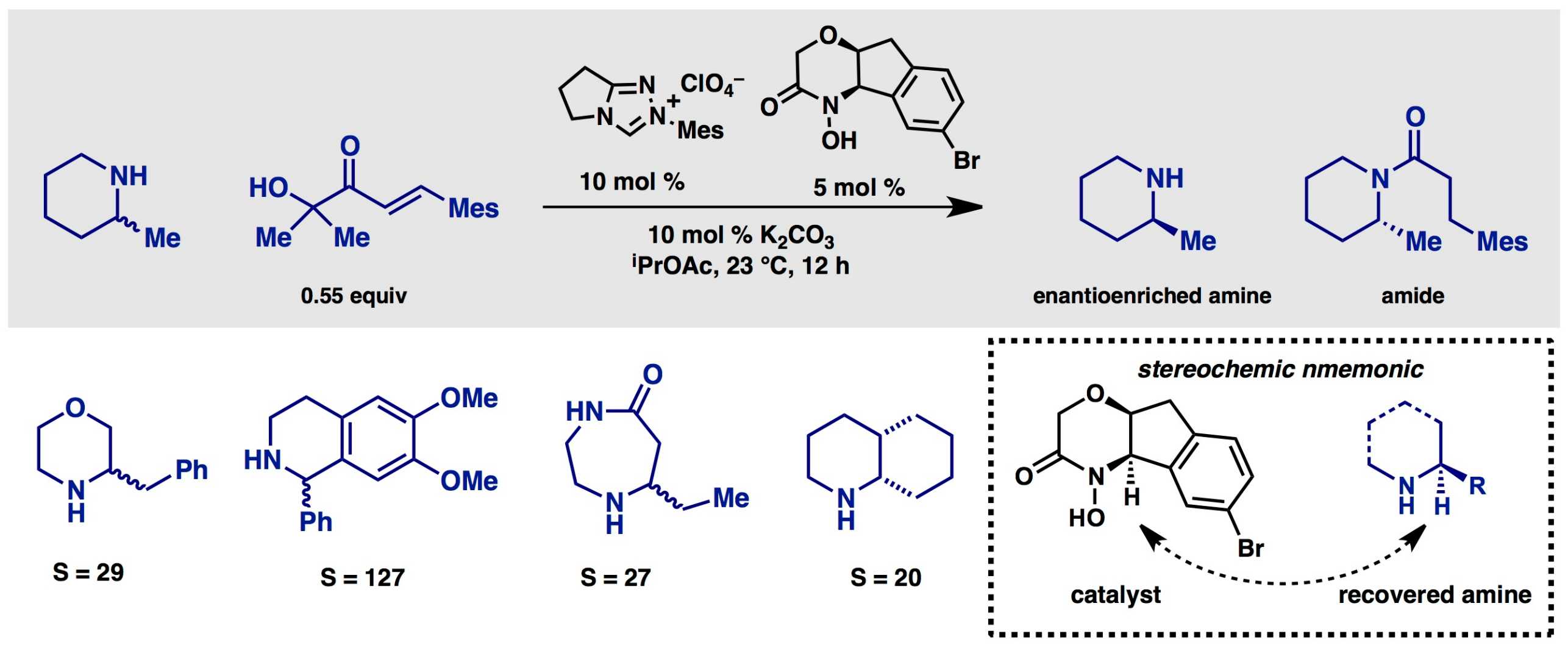
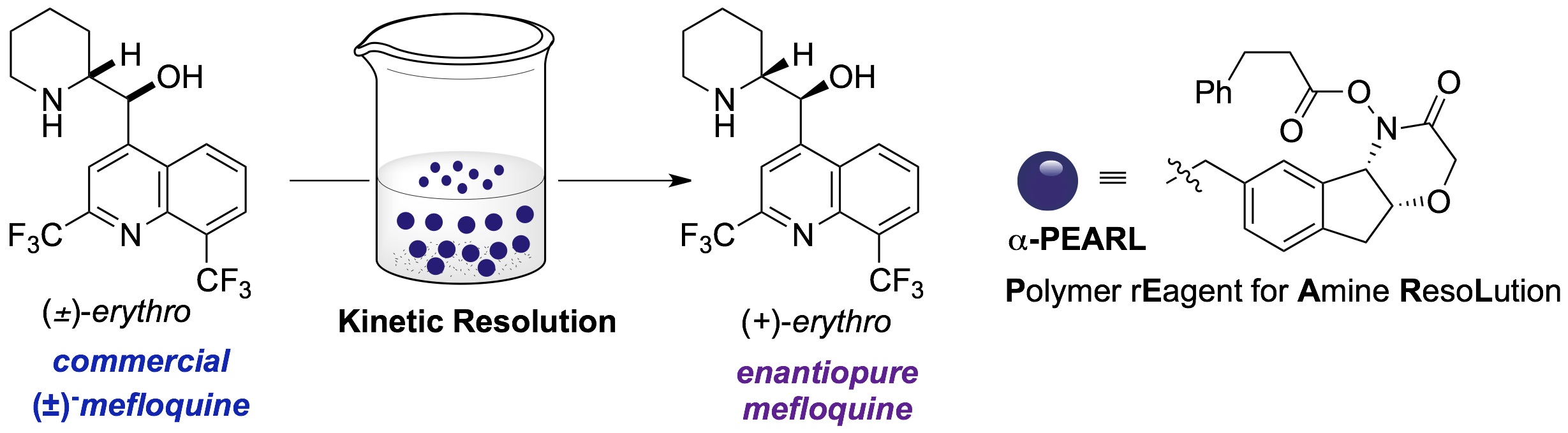
Kinetic resolution of amines
Amines are the most important class of chiral organic compounds in modern pharmaceuticals. Although many can be derived from natural sources or produced by enantioselective reactions, resolution of the enantiomers by chromatography of salt formation remains the state of the art. In order to provide a practical approach to enantiopure amines – particularly chiral, saturated N-hetereocycles – we have developed new reagents and catalysts for the simple kinetic resolution.
Through the innovative design and and synthesis of chiral hydroxamic acids, which serve as enantioselective acylating agents, we have identified both catalytic processes and polymer-bound stoichiometric reagents. These provide access to enantioenriched N-heterocycles such as piperidines, morpholines, piperazines, diazepanes, and tetrahydroisoquinones with good selectivity factors and operationally friendly reaction conditions. In collaboration with Roche, we have developed a reusable resin for amine resolution and applied this to the decagram scale production of the anti-malerial agent (+)-mefloquine from its readily available racemate.
Notable Publications:
(29) Chiang, P.-C.; Kim, Y.; Bode, J. W. external page "Catalytic Amide Formation with α'-Hydroxyenones as Acylating Reagents", Chem. Commun., 2009, 4566–4568.
(53) Binanzer, M.; Hsieh S.-Y.; Bode, J. W. external page "Catalytic Kinetic Resolution of Cyclic Secondary Amines", J. Am. Chem. Soc. 2011, 133, 19698–19701.
(69) Hsieh, S.-Y.; Binanzer, M.; Kreituss, I.; Bode, J. W. external page "Expanded substrate scope and catalyst optimization for the catalytic kinetic resolution of N-heterocycles", Chem. Commun. 2012, 48, 8892–8894.
(74) Kreituss, I.; Murakami, Y.; Binanzer, M.; Bode, J. W. external page "Kinetic Resolution of Nitrogen Heterocycles with a Reusable Polymer-Supported Reagent", Angew. Chem. Int. Ed. 2012, 51, 10660–10663.
(92) Hsieh, S.-Y.; Wanner, B.; Wheeler, P.; Beauchemin, A. M.; Rovis, T; Bode, J. W. external page "Stereoelectronic Basis for the Kinetic Resolution of N-Heterocycles with Chiral Acylating Reagents", Chem. Eur. J. 2014, 20, 7228–7231.
(96) Allen, S. E.; Hsieh, S.-Y.; Gutierrez, O.; Bode, J. W.; Kozlowski, M. C. external page "Concerted Amidation of Activated Esters: Reaction Path and Origins of Selectivity in the Kinetic Resolution of Cyclic Amines via NHC and Hydroxamic Acid Co-Catalyzed Acyl Transfer", J. Am. Chem. Soc. 2014, 136, 11783–11791.
(114) Wanner, B.; Kreituss, I.; Gutierrez, O.; Kozlowski, M. C.; Bode, J. W. external page "Catalytic Kinetic Resolution of Disubstituted Piperidines by Enantioselective Acylation: Synthetic Utility and Mechanistic Insights" J. Am. Chem. Soc. 2015, 137, 11491–11497.
(117) Kreituss, I.; Chen, K.-Y.; Eitel, S. H.; Adam, J.-M.; Wuitschik, G.;Fettes, A.; Bode, J. W. external page "A Robust, Recyclable Resin for Decagram Scale Resolution of (±)-Mefloquine and Other Chiral N-Heterocycles" Angew. Chem. Int. Ed. 2015, Early View (DOI: 10.1002/anie.201509256)
(130) Kreituss, I.; Bode, J. W. external page "Flow chemistry and polymer-supported pseudoenantiomeric acylating agents enable parallel kinetic resolution of chiral saturated N-heterocycles" Nature Chem. 2016, Accepted
(131) Kreituss, I.; Bode, J. W. external page "Catalytic Kinetic Resolution of Saturated N-Heterocycles by Enantioselective Amidation with Chiral Hydroxamic Acids" Acc. Chem. Res. 2016, Accepted